Bromine normally has seven electrons in its valence shell (look at its position relative to the noble gasses on the periodic table, one to the left). In order to become more like a noble gas, the bromine takes on an extra electron, giving it a net negative charge. That means that instead of 7 electrons the bromine has 8 electrons in its valence. Bromine has 7 electrons in its outermost shell which acts as its valence electrons.
What are the quantum numbers for Bromine?

1 Answer
Quantum Numbers define the character of an electron in an energy specific orbital. They do not define an element.
Explanation:


4 Quantum Numbers define the character of an electron in an energy specific orbital.
According to the Pauli Exclusion principle, no two electrons can have the same set of 4 quantum numbers. This is significant in that elements with multiple electrons cannot have more than two in a single orbital.
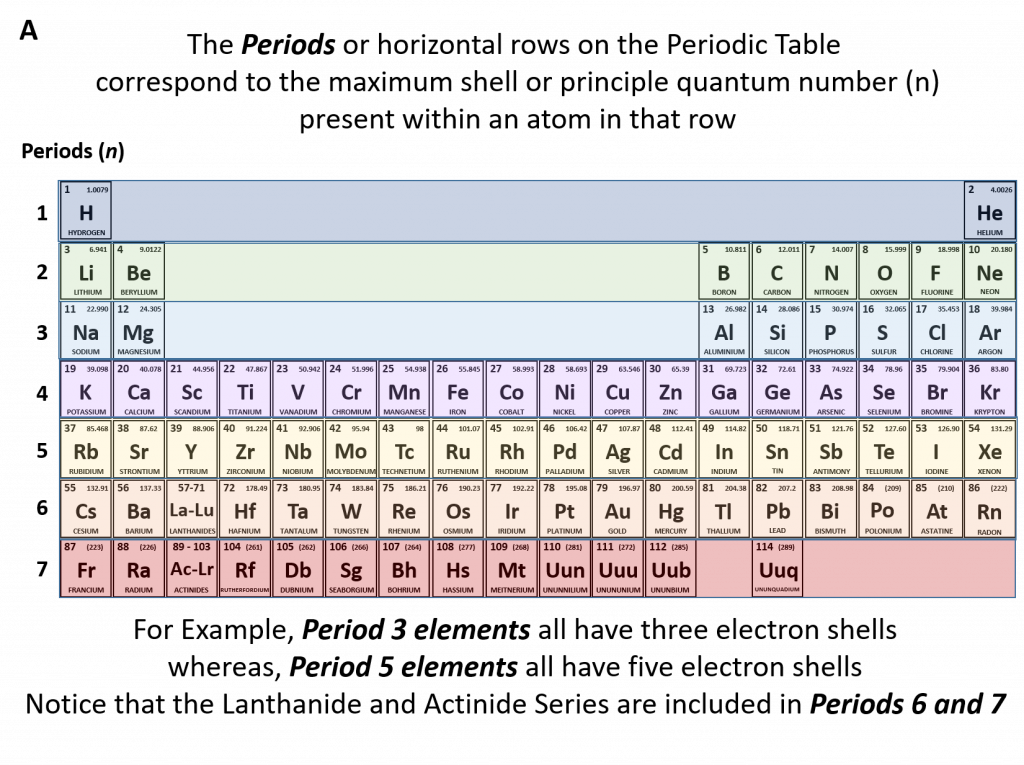
How To Find Number Of Electrons In Bromine

Think of the set of Quantum Numbers for an electron as being a 'discrete energy window' for that electron and no other electron in the element's electron configuration can occupy the same energy window.
The 4 Quantum Numbers (QN) and what they describe are:
- Principle QN (
#n# ) => Defines Principal Energy Level - Orbital QN (
#l# ); also known as Angular Momentum QN => Shape of Orbital - Magnetic QN (
#m_l# ) => Orientation of orbital in 3 dimensions - Spin QN (
#m_s# ) => the spin of the electron, up or down#pm 1/2# , respectively).
The 4 Quantum Numbers for the 'last' electron to fill a Bromine electronic configuration would be the electron in the half-filled
We choose the set of
Number Of Electrons In Boron
Bromine
Related questions
